Qorvo® Biotechnologies’ COVID-19 Rapid Antigen Test Receives FDA Emergency Use Authorization (EUA) for Use in Point-of-Care Settings
BEIJING, China – August 10, 2022 – Qorvo®, Inc. (Nasdaq: QRVO), a leading provider of innovative RF solutions that connect the world, today announced that the U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for Qorvo’s Omnia™ SARS-CoV-2 Antigen Test for use in point-of-care (POC) settings.
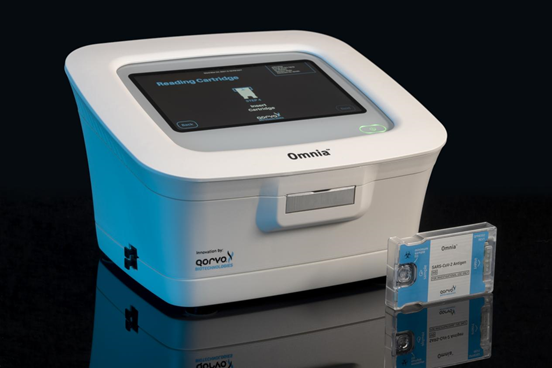
The test is authorized for qualitative testing within six days of symptom onset to determine whether the nucleocapsid viral antigen of SARS-CoV-2 (COVID-19) is present in nasal swab specimens of patients suspected of COVID-19. In addition, the test is also authorized for use in risk groups who do not have suspected COVID-19 symptoms or other epidemiological manifestations. The rule is to test twice within three days, with an interval of no less than 24 hours and no more than 48 hours. Previously, the FDA had issued an emergency use authorization for the test, approving its use in medium and high-complexity environments such as laboratories. This emergency use authorization expands Qorvo's market beyond the laboratory, including doctors' offices, urgent care centers, retail pharmacies, employee health testing, and any other place where Clinical Laboratory Improvement Amendments (CLIA) exempt testing can be performed.
“As COVID-19 testing becomes more widely available and the Omicron variant shows lower viral loads, point-of-care settings require high-quality rapid testing infrastructure,” said Erik Allen, Qorvo’s newly appointed vice president and president of Qorvo Biotechnologies. “The Qorvo Omnia platform offers a unique combination of performance, automated workflow and scalability to efficiently meet the needs of point-of-care testing.”
The Qorvo Omnia platform uses innovative diagnostic technology to rapidly produce highly sensitive and specific COVID-19 test results on an easy-to-use standalone platform using high-frequency bulk acoustic wave (BAW) sensors . BAW sensor technology can achieve low limits of detection (LOD) levels that approximate molecular detection capabilities. As viral loads decrease, especially with the emergence of Omicron variants and subtypes, low viral loads in patient samples have challenged existing over-the-counter (OTC) lateral flow tests. The Qorvo Omnia platform has the technical advantage to continuously and accurately detect COVID-19 related antigens during the peak period of Omicron.
Qorvo's Omnia platform performs well in terms of limit of detection (LOD) compared to PCR methods. The Omnia platform demonstrated 85% sensitivity (PPA) during clinical studies, supporting emergency use authorization of the device for point-of-care use; this project was funded by a grant from the National Institutes of Health (NIH) and officially launched in the second half of 2021. During the widespread spread of the Omicron variant in early 2022, the NIH funded an independent study. In this study, when the sample reached or exceeded the detection limit of the comparative PCR method, Qorvo still performed well, with a sensitivity of 86%. In all relevant studies, the specificity reached 100%.
"Today, as testing needs evolve with the emergence of Omicron variants, Qorvo's antigen tests ensure the performance levels our customers need in a variety of testing scenarios," said Peter Matos, president and CEO of healthcare consulting firm Traeokos.
This project was funded in whole or in part by federal funds from the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health, within the U.S. Department of Health and Human Services, under Contract No. 75N92021C00008.
In April 2021, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for Qorvo Omnia’s SARS-CoV-2 Antigen Test for use in moderate- and high-complexity settings, and from July 2022 also in point-of-care settings.
The Qorvo Omnia SARS-CoV-2 Antigen Test has not been cleared or approved by the FDA. It is authorized by the FDA under the Emergency Use Authorization regulations for performance of exempt, moderate, or high complexity testing only by laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 USC §263a. The tests are authorized only for the detection of proteins from SARS-CoV-2 and not for any other viruses or pathogens. These tests are authorized for use only for the period of time that the emergency use of in vitro diagnostic tests for the detection and/or diagnosis of COVID-19 is warranted pursuant to section 564(b)(1) of the Emergency Use Authorization Act, 21 USC § 360bbb-3(b)(1), unless such authorization is terminated or revoked sooner.
Previous article:Flexible semiconductors make wearable neuromorphic chips
Next article:IAR Systems supports Osong Medical Innovation Foundation (KBIO Health) in developing advanced medical devices
Recommended ReadingLatest update time:2024-11-15 13:24



- Popular Resources
- Popular amplifiers
-
 FOUNDRY PROCESS QUALIFICATION GUIDELINES – TECHNOLOGY QUALIFICATION VEHICLE TESTING JEP001-3B
FOUNDRY PROCESS QUALIFICATION GUIDELINES – TECHNOLOGY QUALIFICATION VEHICLE TESTING JEP001-3B -
 A review of learning-based camera and lidar simulation methods for autonomous driving systems
A review of learning-based camera and lidar simulation methods for autonomous driving systems -
 Evaluating Roadside Perception for Autonomous Vehicles: Insights from Field Testing
Evaluating Roadside Perception for Autonomous Vehicles: Insights from Field Testing -
 Autonomous driving and advanced driver assistance systems (ADAS): applications, development, legal issues and testing
Autonomous driving and advanced driver assistance systems (ADAS): applications, development, legal issues and testing
- High-speed 3D bioprinter is available, using sound waves to accurately build cell structures in seconds
- [“Source” Observation Series] Application of Keithley in Particle Beam Detection Based on Perovskite System
- STMicroelectronics’ Biosensing Innovation Enables Next-Generation Wearable Personal Healthcare and Fitness Devices
- China's first national standard for organ chips is officially released, led by the Medical Devices Institute of Southeast University
- The world's first non-electric touchpad is launched: it can sense contact force, area and position even without electricity
- Artificial intelligence designs thousands of new DNA switches to precisely control gene expression
- Mouser Electronics provides electronic design engineers with advanced medical technology resources and products
- Qualcomm Wireless Care provides mobile terminal devices to empower grassroots medical workers with technology
- Magnetoelectric nanodiscs stimulate deep brain noninvasively
- LED chemical incompatibility test to see which chemicals LEDs can be used with
- Application of ARM9 hardware coprocessor on WinCE embedded motherboard
- What are the key points for selecting rotor flowmeter?
- LM317 high power charger circuit
- A brief analysis of Embest's application and development of embedded medical devices
- Single-phase RC protection circuit
- stm32 PVD programmable voltage monitor
- Introduction and measurement of edge trigger and level trigger of 51 single chip microcomputer
- Improved design of Linux system software shell protection technology
- What to do if the ABB robot protection device stops
- Wi-Fi 8 specification is on the way: 2.4/5/6GHz triple-band operation
- Wi-Fi 8 specification is on the way: 2.4/5/6GHz triple-band operation
- Vietnam's chip packaging and testing business is growing, and supply-side fragmentation is splitting the market
- Vietnam's chip packaging and testing business is growing, and supply-side fragmentation is splitting the market
- Three steps to govern hybrid multicloud environments
- Three steps to govern hybrid multicloud environments
- Microchip Accelerates Real-Time Edge AI Deployment with NVIDIA Holoscan Platform
- Microchip Accelerates Real-Time Edge AI Deployment with NVIDIA Holoscan Platform
- Melexis launches ultra-low power automotive contactless micro-power switch chip
- Melexis launches ultra-low power automotive contactless micro-power switch chip
- Share an open source stepper motor controller + PID algorithm + high-precision magnetic angle detection
- The microcontroller uses C language to generate sine wave DA data
- Prize-winning live broadcast: Registration for Novatek security surveillance solutions and future technology directions has begun!
- [Chuanglong TLA40i-EVM development board] +02. Power on, CPU and DDR test (zmj)
- Unpacking the GD32L233C-START evaluation board and downloading related resources
- New design rules after AD14...
- Characteristics and selection of common protection components for lightning overvoltage protection
- Failures in the functional safety concept
- Apple launches self-service repair program, you can repair your phone yourself in the future
- How to prevent PCB board from bending and warping during reflow oven

 FOUNDRY PROCESS QUALIFICATION GUIDELINES – TECHNOLOGY QUALIFICATION VEHICLE TESTING JEP001-3B
FOUNDRY PROCESS QUALIFICATION GUIDELINES – TECHNOLOGY QUALIFICATION VEHICLE TESTING JEP001-3B















 京公网安备 11010802033920号
京公网安备 11010802033920号