The biggest obstacle to the promotion of hydrogen fuel cell vehicles is the high price and rare production of platinum. This expensive metal is particularly important for the redox reaction at the cathode of the fuel cell. At the cathode, protons that pass through the polymer membrane, oxygen atoms in the air, and electrons transmitted by the wire combine to form the product - water.
Recently, a team of chemists and materials scientists from the U.S. Department of Energy's Lawrence Berkeley National Laboratory (LBNL) and Argonne National Laboratory (ANL) have developed an innovative three-dimensional "nanoframe" catalyst that outperforms conventional platinum-carbon particle catalysts in catalyzing anodic oxidation reactions, an unprecedented level of performance and even far exceeds the U.S. Department of Energy's estimate that the technology may reach the technical level in 2017.
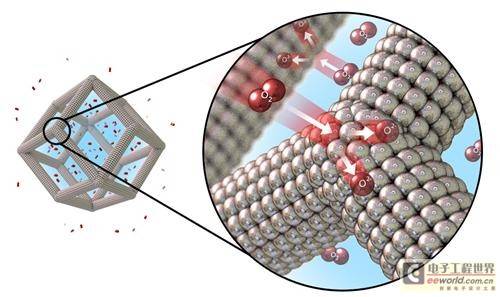
This bimetallic catalyst is composed of platinum and nickel. It is hollow, highly active, and has large inner and outer surface sizes, making it much more efficient and cost-effective than current catalyst products.
The catalyst can also work in alkaline hydrolysis cells, splitting water into oxygen and hydrogen. Depending on the amount of electricity consumed, it could also be used as a hydrogen production device in the future. The alkaline hydrolysis cell contains two electrodes separated by a membrane, which are immersed in an alkaline electrolyte composed of potassium hydroxide. The researchers compared the performance of the new catalyst with that of a conventional platinum-carbon catalyst in the cell and found that the new catalyst performed an order of magnitude better.
In recent years, research around the world has focused on alloying platinum with other cheaper metals to form alloys, while reducing the price of catalysts while maintaining performance. Another improvement method is to develop hollow, cage-shaped, porous materials to add smaller amounts of precious metal catalysts. Peidong Yang, a chemist from the LBNL laboratory and a professor at the University of California, Berkeley, who led the project, said that this unconventional geometric structure makes it easier to quantitatively improve physical and chemical properties.
New bimetallic nanocage
Peidong Yang said: "We started studying the dissolution process of nanoparticle catalysts ten years ago. At first, we focused on single elements such as platinum, analyzing the effects of size and shape on catalyst performance. But as the research progressed, we paid more attention to bimetallic catalysts, such as platinum-nickel and platinum-copper. Three or four years ago, two of my postdocs observed an unexpected phenomenon when they put a platinum-nickel sample into a solvent: they found that the bimetallic nanoparticle sample gradually formed a new morphology after two weeks.
This was unexpected for us, but we knew we were on to something when we saw that the surface of the 3D nanoframe was completely covered with catalytic sites.”
The Berkeley researchers then looked through existing literature and found that Vojislav Stamenkovic, a chemist at the ANL laboratory, had done quite a bit of research on large-capacity single-crystal catalyst substrates. Based on Vojislav's research, it was concluded that bimetallic materials would also be promising electrochemical catalysts. So we contacted him and started a collaboration.
Hollow structure
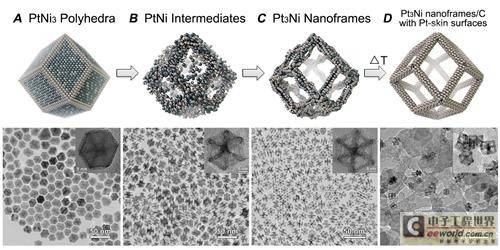
The study found that the starting material in the solution - solid crystalline PtNi3 polyhedron will be transformed into a PtNi intermediate material, and then through internal corrosion, it will be transformed into a Pt3Ni nanoframe through which oxygen atoms can pass. The edges of the PtNi3 polyhedron are composed of high concentrations of platinum, which are also retained in the subsequent Pt3Ni nanoframe. The initial polyhedron is composed of three nickel atoms and one platinum atom, while the final nanoframe is just the opposite.
"Polyhedra are a common type of nanostructure that have been used in catalyst research for many years," Stamenkovic said. "But our study shows that other approaches are possible. With the framework, we completely open up the structure and get rid of the atoms buried inside the material that just take up volume but have no function. In the end, there are still a significant number of catalytic sites left, located all over the surface of the framework and accessible from many directions."
The solvent containing oxygen causes a dissolution effect inside the polyhedron, eventually forming a hollow dodecahedral nanoframe structure. By increasing the reaction temperature, the reaction time was shortened from two weeks to 12 hours.
After completing the preparation of the basic raw materials, the researchers wanted to verify the stability of the material in the harsh electrochemical environment of the fuel cell stack, so they wrapped a "protective layer" around the platinum atoms of the nanoframe to improve its durability. By annealing the nanoframe in argon, a "protective layer" composed of platinum was produced on the surface of the nanoframe.
"We speculate that the oxygen atoms brought the nickel nanoparticles to the framework, and the annealing effect of the argon gas brought the platinum to the surface," Yang said.
Ultra-high activity catalyst
The inner and outer surfaces of the Pt3Ni framework are composed of nano-platinum structures, which improve the activity of redox reactions. Compared with the best platinum-carbon catalyst, the mass activity and specific activity of the nano-frame catalyst are increased by 36 times and 22 times respectively. The new material has not yet been put into actual fuel cell stacks for testing.
"Compared to other attempts to synthesize hollow nanostructures artificially, our method does not require electricity or strong oxidants, but proceeds spontaneously in air," Yang said. "The open structure of the platinum-nickel nanoframes satisfies some of the principles in the design of advanced nanodot catalysts, such as high aspect ratio, good three-dimensional proximity, and less precious metal usage."
This new catalyst significantly reduces the amount of platinum used, and the next generation of low-cost, high-performance exhaust catalysts is likely to be developed from it.
The artificial synthesis process of the catalytic material can be easily scaled up to meet the needs of practical applications in grams. Importantly, the method can be further extended to the synthesis of other alloy nanoframes, such as platinum-cobalt alloys, platinum-copper alloys, platinum-rhenium-nickel alloys, and platinum-lead-nickel alloys.
Previous article:The battery of the future will be like this: fully charged in a few seconds and standby for months
Next article:Power battery material competition: BYD manganese vs Tesla graphene
- Popular Resources
- Popular amplifiers
- Car key in the left hand, liveness detection radar in the right hand, UWB is imperative for cars!
- After a decade of rapid development, domestic CIS has entered the market
- Aegis Dagger Battery + Thor EM-i Super Hybrid, Geely New Energy has thrown out two "king bombs"
- A brief discussion on functional safety - fault, error, and failure
- In the smart car 2.0 cycle, these core industry chains are facing major opportunities!
- The United States and Japan are developing new batteries. CATL faces challenges? How should China's new energy battery industry respond?
- Murata launches high-precision 6-axis inertial sensor for automobiles
- Ford patents pre-charge alarm to help save costs and respond to emergencies
- New real-time microcontroller system from Texas Instruments enables smarter processing in automotive and industrial applications
- Innolux's intelligent steer-by-wire solution makes cars smarter and safer
- 8051 MCU - Parity Check
- How to efficiently balance the sensitivity of tactile sensing interfaces
- What should I do if the servo motor shakes? What causes the servo motor to shake quickly?
- 【Brushless Motor】Analysis of three-phase BLDC motor and sharing of two popular development boards
- Midea Industrial Technology's subsidiaries Clou Electronics and Hekang New Energy jointly appeared at the Munich Battery Energy Storage Exhibition and Solar Energy Exhibition
- Guoxin Sichen | Application of ferroelectric memory PB85RS2MC in power battery management, with a capacity of 2M
- Analysis of common faults of frequency converter
- In a head-on competition with Qualcomm, what kind of cockpit products has Intel come up with?
- Dalian Rongke's all-vanadium liquid flow battery energy storage equipment industrialization project has entered the sprint stage before production
- Allegro MicroSystems Introduces Advanced Magnetic and Inductive Position Sensing Solutions at Electronica 2024
- Car key in the left hand, liveness detection radar in the right hand, UWB is imperative for cars!
- After a decade of rapid development, domestic CIS has entered the market
- Aegis Dagger Battery + Thor EM-i Super Hybrid, Geely New Energy has thrown out two "king bombs"
- A brief discussion on functional safety - fault, error, and failure
- In the smart car 2.0 cycle, these core industry chains are facing major opportunities!
- The United States and Japan are developing new batteries. CATL faces challenges? How should China's new energy battery industry respond?
- Murata launches high-precision 6-axis inertial sensor for automobiles
- Ford patents pre-charge alarm to help save costs and respond to emergencies
- New real-time microcontroller system from Texas Instruments enables smarter processing in automotive and industrial applications
- 【I-Prober 520】Unboxing and simple review
- EEWORLD University Hall----Live Replay: Maxim supports integrated digital IO technology for industrial systems
- Want to buy second-hand AM335x Starter Kit development board
- Emulating I2C communication using GPIO on C2000
- 【TouchGFX Design】Make a Rubik's Cube
- Two circuits for modeling the behavior of a two-input AND gate
- Circuit protection devices protect mobile devices from ESD
- Let's discuss some gadgets commonly used by RF engineers
- MSP430 interrupt vector table
- For thyristors, can digital resistors be used to control chopping in addition to optocouplers?


 LM49450SQ/NOPB
LM49450SQ/NOPB











 京公网安备 11010802033920号
京公网安备 11010802033920号