Flow cytometer is a device for automatic analysis and sorting of cells. It can quickly measure, store and display a series of important biophysical and biochemical characteristic parameters of dispersed cells suspended in liquid, and can sort out designated cell subpopulations according to the pre-selected parameter range. Most flow cytometers are zero-resolution instruments, which can only measure indicators such as total nucleic acid amount and total protein amount of a cell, but cannot identify and measure the amount of nucleic acid or protein in a specific part. In other words, its detail resolution is zero.

Figure 1 Flow cytometer
working principle
The following is a brief introduction to the working principles of flow cytometer-related parameter measurement, sample sorting and data processing.
Parameter measurement principle
Flow cytometers can measure multiple parameters simultaneously, and the information mainly comes from specific fluorescence signals and non-fluorescence scattering signals. The measurement is carried out in the measurement area, which is the vertical intersection of the irradiating laser beam and the liquid flow beam ejected from the nozzle. When a single cell in the center of the liquid flow passes through the measurement area, it will scatter light to the entire space with a solid angle of 2π when irradiated by the laser, and the wavelength of the scattered light is the same as the wavelength of the incident light. The intensity of scattered light and its spatial distribution are closely related to the size, morphology, plasma membrane and internal structure of the cell, because these biological parameters are related to the optical properties of the cell such as reflection and refraction of light. Cells that have not suffered any damage have characteristic scattering of light, so different scattered light signals can be used to analyze and sort unstained living cells. Due to the change in optical properties, the scattered light signals of fixed and stained cells are of course different from those of living cells. Scattered light is not only related to the parameters of the cell as the scattering center, but also to non-biological factors such as the scattering angle and the solid angle of collecting scattered light.
In flow cytometry, two types of scattered light measurements are commonly used: ① forward angle (i.e. 0 angle) scattering (FSC); ② side scattering (SSC), also known as 90-angle scattering. The angle here refers to the approximate angle between the direction of laser beam irradiation and the axial direction of the photomultiplier tube that collects the scattered light signal. Generally speaking, the intensity of forward angle scattered light is related to the size of the cell. For the same cell population, it increases with the increase of the cell cross-sectional area; for spherical living cells, experiments have shown that it is basically linearly related to the cross-sectional area within a small solid angle range; for cells with complex shapes and orientations, the difference may be very large, and special attention should be paid. The measurement of side scattered light is mainly used to obtain relevant information about the particle properties of the fine structure inside the cell. Although side scattered light is also related to the shape and size of the cell, it is more sensitive to the refractive index of the cell membrane, cytoplasm, and nuclear membrane, and can also give a sensitive response to larger particles in the cytoplasm.
In actual use, the instrument first measures the light scattering signal. When light scattering analysis is used in conjunction with fluorescent probes, stained and unstained cells in the sample can be identified. The most effective use of light scattering measurement is to identify certain subpopulations from a heterogeneous population.
Fluorescence signals mainly include two parts: ① Autofluorescence, that is, the fluorescence emitted by fluorescent molecules inside cells after being irradiated with light without fluorescent staining; ② Characteristic fluorescence, that is, the fluorescence emitted by fluorescent dyes bound to cells after being irradiated with light. Its fluorescence intensity is weak and its wavelength is different from that of the irradiating laser. Autofluorescence signals are noise signals, which will interfere with the resolution and measurement of specific fluorescence signals in most cases. In measurements such as immunocytochemistry, for fluorescent antibodies with low binding levels, how to improve the signal-to-noise ratio is a key. Generally speaking, the higher the content of molecules that can produce autofluorescence (such as riboflavin, cytochrome, etc.) in cell components, the stronger the autofluorescence; the higher the ratio of dead cells to live cells in cultured cells, the stronger the autofluorescence; the higher the proportion of bright cells contained in the cell sample, the stronger the autofluorescence.
The main measures to reduce the interference of autofluorescence and improve the signal-to-noise ratio are: ① Try to use brighter fluorescent dyes; ② Choose appropriate laser and filter optical systems; ③ Use electronic compensation circuits to compensate for the background contribution of autofluorescence.
Sample sorting principle
The sorting function of the flow cytometer is completed by the cell sorter. The overall process is: the liquid column ejected from the nozzle is divided into a series of small droplets, and the logic circuit determines whether it will be sorted according to a selected parameter, and then the charging circuit charges the selected cell droplets. The charged droplets carry the cells through the electrostatic field and are deflected and fall into the collector; other liquids are sucked away as waste liquid. Some types of instruments also use capture tubes for sorting.
Stable small droplets are formed by the piezoelectric crystal on the flow chamber vibrating under the action of an electrical signal of tens of KHz, forcing the liquid flow to break evenly. Generally, the distance between droplets is about hundreds of μm. The experimental empirical formula f=v/4.5d gives the frequency of the oscillation signal for forming stable water droplets. Among them, v is the liquid flow velocity and d is the diameter of the nozzle. It can be seen that the use of nozzles with different apertures and changes in the liquid flow velocity may change the sorting effect. The deflection of the sorted cell-containing droplets in the electrostatic field is completed by the charging circuit and the deflection plate. The charging voltage is generally selected as +150V, or -150V; the potential difference between the deflection plates is several thousand volts. The charging pulse generator in the charging circuit is controlled by a logic circuit, so it takes a delay time from parameter measurement to logic selection to pulse charging, which is generally tens of ms. Accurately measuring the delay time is the key to determining the quality of sorting. The instrument mostly uses a shift register digital circuit to generate the delay. It can be appropriately adjusted according to specific requirements.
(50) Data processing principle: FCM data processing mainly includes data display and analysis. How to interpret the results given by the instrument depends on the specific problem to be solved.
① Data display: FCM's data display modes include single parameter histogram, two-dimensional dot plot, two-dimensional contour plot, pseudo three-dimensional plot and list mode.
Histogram is the most commonly used graphical display form for one-dimensional data. It can be used for both qualitative and quantitative analysis, and is similar to the curve given by a general XY plane tracer. Depending on the type of amplifier selected, the coordinates can be linear or logarithmic, expressed in "channel numbers", which is essentially the intensity of the measured fluorescence or scattered light. The coordinates generally represent the relative number of cells. Figure 2 shows a histogram. Its limitation is that it can only display the relationship between one parameter and cells.
The two-dimensional dot plot can show the relationship between two independent parameters and the relative number of cells. Coordinates and coordinates are two independent parameters related to cells, and each point on the plane indicates the existence of cells with corresponding coordinate values (Figure 3). Two one-dimensional histograms can be obtained from the two-dimensional dot plot, but due to the existence of the merger phenomenon, the amount of information in the two-dimensional dot plot is greater than that of the two one-dimensional histograms. The so-called merger means that multiple cells with the same two-dimensional coordinates are only shown as one point on the graph, so it is difficult to show its fine structure in places where the cell points are densely packed.
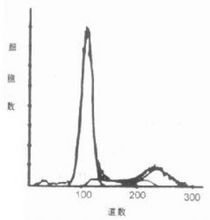
Figure 2 Histogram
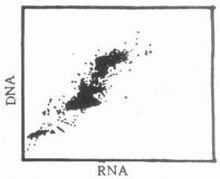
Figure 3 Two-dimensional point diagram
The two-dimensional contour map is similar to the contour line representation on the map. It is a display method set up to overcome the shortcomings of the two-dimensional point map. Each continuous curve on the contour map has the same relative or absolute number of cells, that is, "equal height". The higher the level of the curve, the more cells it represents. Generally, the intervals of the number of cells represented by the level are equal, so the denser the contour lines, the greater the rate of change, and the sparser the contour lines, the more balanced the change. Figure 4 shows the style of a two-dimensional contour map.
The pseudo three-dimensional map is a visually intuitive method of expressing the two-dimensional contour map using computer technology. It simultaneously displays the implicit coordinates and cell numbers in the original two-dimensional map, but the parameter dimension map can be rotated, tilted, etc., so as to observe the structure and details of the "peaks" and "valleys" from multiple angles, which is undoubtedly helpful for data analysis. Figure 5 is a schematic diagram of the pseudo three-dimensional map.
Previous article:Basic principles and technical indicators comparison of feces analyzers
Next article:Principle, classification and comparison of several amino acid analyzers
- Popular Resources
- Popular amplifiers
- New IsoVu™ Isolated Current Probes: Bringing a New Dimension to Current Measurements
- Modern manufacturing strategies drive continuous improvement in ICT online testing
- Methods for Correlation of Contact and Non-Contact Measurements
- Keysight Technologies Helps Samsung Electronics Successfully Validate FiRa® 2.0 Safe Distance Measurement Test Case
- From probes to power supplies, Tektronix is leading the way in comprehensive innovation in power electronics testing
- Seizing the Opportunities in the Chinese Application Market: NI's Challenges and Answers
- Tektronix Launches Breakthrough Power Measurement Tools to Accelerate Innovation as Global Electrification Accelerates
- Not all oscilloscopes are created equal: Why ADCs and low noise floor matter
- Enable TekHSI high-speed interface function to accelerate the remote transmission of waveform data
- Intel promotes AI with multi-dimensional efforts in technology, application, and ecology
- ChinaJoy Qualcomm Snapdragon Theme Pavilion takes you to experience the new changes in digital entertainment in the 5G era
- Infineon's latest generation IGBT technology platform enables precise control of speed and position
- Two test methods for LED lighting life
- Don't Let Lightning Induced Surges Scare You
- Application of brushless motor controller ML4425/4426
- Easy identification of LED power supply quality
- World's first integrated photovoltaic solar system completed in Israel
- Sliding window mean filter for avr microcontroller AD conversion
- What does call mean in the detailed explanation of ABB robot programming instructions?
- STMicroelectronics discloses its 2027-2028 financial model and path to achieve its 2030 goals
- 2024 China Automotive Charging and Battery Swapping Ecosystem Conference held in Taiyuan
- State-owned enterprises team up to invest in solid-state battery giant
- The evolution of electronic and electrical architecture is accelerating
- The first! National Automotive Chip Quality Inspection Center established
- BYD releases self-developed automotive chip using 4nm process, with a running score of up to 1.15 million
- GEODNET launches GEO-PULSE, a car GPS navigation device
- Should Chinese car companies develop their own high-computing chips?
- Infineon and Siemens combine embedded automotive software platform with microcontrollers to provide the necessary functions for next-generation SDVs
- Continental launches invisible biometric sensor display to monitor passengers' vital signs
- Analyze the cause of tms320c6455 burning
- Beacon navigation sound positioning and recognition - microphone array
- How to make a boost circuit so that the output is equal to the input voltage?
- An experienced person explains Shannon's theorem, Nyquist's theorem, coding and modulation
- Suggested angles for disassembling products (for reference by netizens who participate in disassembly activities in the community)
- SoC chip debugging experience
- Self-powered switch realizes ZigBee transmission
- Dynamic power management based on Linux: making embedded devices more energy-efficient
- CLUE development board information
- What is the difference between a rectifier circuit and a detector circuit?



 LTC1540IS8#TRPBF
LTC1540IS8#TRPBF











 京公网安备 11010802033920号
京公网安备 11010802033920号